Type Of Media:学術論文
Publication/Magazine/Media:米国化学会誌 J. Am. Chem. Soc.
Author:Chun Yin Jerry Lau, Hiroaki Kinoh, Xueying Liu, Jiayuan Feng, Fadlina Aulia, Kaori Taniwaki, Nan Qiao, Satomi Ogura, Mitsuru Naito and Kanjiro Miyata
Structurally Dynamic Polyplexes Enhance Sentinel Lymph Node Delivery of Antisense Oligonucleotides to Inhibit Breast Cancer Recurrence and Metastasis
Abstract:
Innovation Center of NanoMedicine (Center Director: Kazunori Kataoka, Location: Kawasaki-Japan, Abbreviation: iCONM) has summarized the results of its collaborative research with Professor Kanjiro Miyata's group from the Department of Materials Engineering/Bioengineering at the Graduate School of Engineering, The University of Tokyo (visiting researcher at iCONM). The paper titled " Structurally dynamic polyplexes enhance sentinel lymph node delivery of antisense oligonucleotides to inhibit breast cancer recurrence and metastasis" has been published in Journal of the American Chemical Society on June 20.
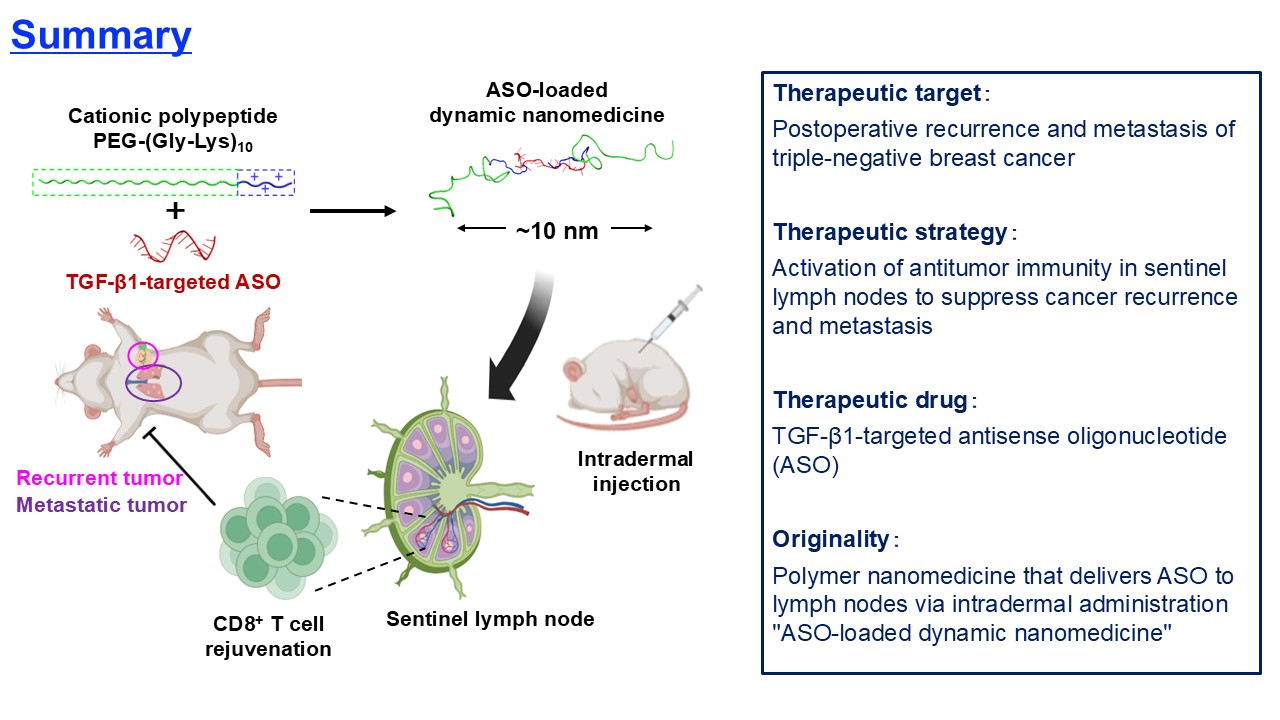
Sentinel lymph nodes (SLNs) serve as the first checkpoint in breast cancer metastasis and play a crucial role in halting cancer progression. However, in advanced cancers with metastatic potential, cytotoxic CD8-positive T cells, which are supposed to attack cancer cells, are inactivated through the protein TGF-β1 secreted by cancer cells in the SLN. This study aimed to reactivate these cytotoxic CD8-positive T cells to suppress the recurrence and metastasis of breast cancer. Specifically, nanomachines were strategically designed to deliver "Antisense nucleic acid (ASO)" aimed at lowering the expression level of TGF-β1 in the SLN.
Generally, biological tissues, including lymph nodes, have fine mesh structures, requiring the preparation of drugs or drug delivery systems that can pass through these structures. Compared to the vascular system, delivering drugs via the lymphatic system poses greater challenges. For example, lipid nanoparticles used in COVID-19 vaccines are approximately 100 nm in size, a relatively large size that might hinder their entry into lymph nodes. In contrast, the research team successfully created "dynamic nanomachines" that are 10 nm in size, capable of loosely encapsulating ASOs to efficiently deliver them to SLNs and effectively engage with target immune cells.
This nanomachine was achieved by precisely adjusting the "positively charged amino acid sequences" of block polymers and the length (or molecular weight) of the biocompatible polymer polyethylene glycol (PEG). Specifically, it was found that an amino acid structure consisting of a repeat of glycine and ten lysines, which is neutral, can loosely encapsulate negatively charged ASOs and release them effectively within target cells. Additionally, adjusting the molecular weight of PEG to around 10,000 increased the ASO distribution within the SLN while reducing its distribution to other normal tissues. Experimental results demonstrated that the optimized nanomachines reduced TGF-β1 levels in SLNs, reactivated depleted CD8-positive T cells within SLNs, and dramatically suppressed cancer recurrence and lung metastasis after breast cancer resection in mouse models. These findings provide molecular design guidelines for simple and safe nucleic acid therapies for advanced breast cancer. Overall, this study presents a promising approach to suppress metastasis and recurrence of refractory breast cancers, such as triple-negative breast cancer (TNBC), for which there are currently no effective treatments.
https://doi.org/10.1021/jacs.5c04234